

So the magic words "atom smasher" were used by Kaku himself, in his book on String theory "Hyperphysics" (and possibly that is an apt description).īut when it was plugged in, it knocked out many domestic fuses in the surrounding neighborhood. If you were to walk by my atom smasher, it would pull the fillings out of your teeth-that’s how powerful the magnet was going to be.” Well, the magnetic field was so powerful-about 20,000 times the Earth’s magnetic field. My father grabbed it, ran to the goalpost and we wound 22 miles of copper wire on the football field. She ran with this strand of wire to the 50-yard line. The wire was so heavy, I put the wire on the goal post and I gave it to my mother. I got 400 pounds of transformer steel, 22 miles of copper wire, and built a 2.3-million electron-volt betatron in the garage. It's more like a super Cathode Ray Tube than any sort of mini LHC.Īnd then I went to Westinghouse.
#Build an atom simulator#
Create your atom with this simulator and learn chemistry, enjoy science. With the help of the periodic table you can build an atom of the chemical element of your choice. Chemical reactions largely involve atoms or groups of atoms and the interactions between their electrons.The device is hyped up a bit by articles such as Astounding School project. Build an atom is an online atom building simulator, an interactive activity in which you can add protons, neutrons and electrons to create stable atoms and ions. In general, electrons are easier to add or remove from an atom than a proton or neutron.

Explore the Build an Atom simulation with your group.
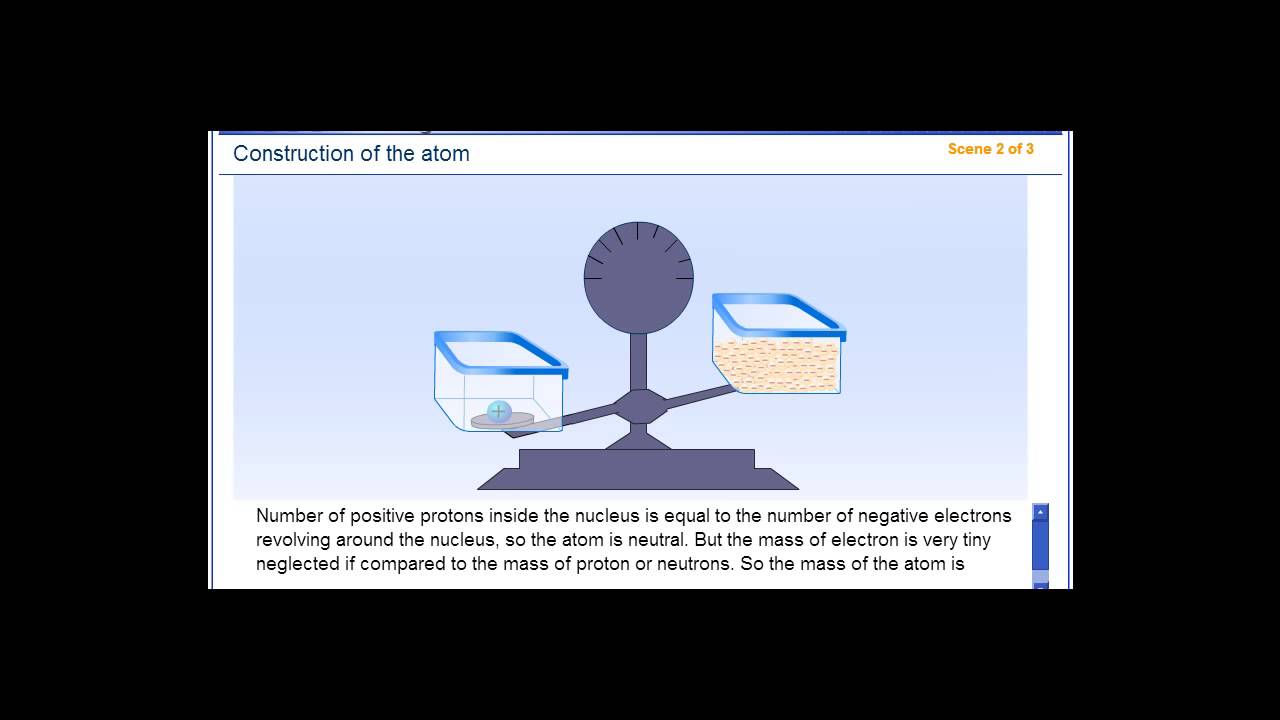
The average size of an atom is about 100 picometers or one ten-billionth of a meter. Electrons can also move between orbitals. Technically, an electron can be found anywhere within the atom, but spends most of its time in the region described by an orbital. Some electron shells resemble spheres, but others look more like dumb bells or other shapes. Simple models show electrons orbiting the nuclear in a near-circular orbit, like planets orbiting a star, but real behavior is much more complex. Electrons are organized into shells, which is a region where an electron is most likely found. Electrons move around outside the nucleus.The nucleus carries a positive electrical charge. The nucleus of an atom contains protons and neutrons.The mass of a proton is 1840 times greater than the mass of an electron. The mass of a proton is essentially the same as that of a neutron. Protons and neutrons are about the same size as each other and are much larger than electrons.

With a.2 answers Top answer: The device is hyped up a bit by articles such as Astounding School project. In other words, neutrons do not have a charge and are not electrically attracted to either electrons or protons. A Tesla coil, simple rf bottle ion source, and a vacuum beamline can certainly be built by reasonably competent folks in their garage. Electrons and protons are electrically attracted to each other. Like charges (protons and protons, electrons and electrons) repel each other. The charge of a proton and an electron are equal in magnitude, yet opposite in sign. You'll start with a hydrogen atom, which contains one proton and one electron.


 0 kommentar(er)
0 kommentar(er)
